Back when I was doing my medical physics residency (oh about 6 or so years ago now), I was asked to evaluate a triple-energy window technique for scatter reduction in nuclear medicine imaging. I stashed a copy of what I wrote up over on my other much neglected website and just thought I’d move it over here for storage and posterity. It’s pretty rough and not anywhere near any kind of publishable state, but it was fun to work on and provided a good learning experience.
Objective
To test and evaluate the usefulness of the Triple Energy Window (TEW) method for scatter correction proposed by Ogawa et al.
Background
In a scatter correction method proposed by Ogawa et al1, the number of scattered photons in each pixel is estimated using two energy windows adjacent to the main photopeak window. The number of scattered photons in the photopeak window is determined by calculating the area of the trapezoid underneath the line joining the two scatter windows in the scatter spectrum.
For each pixel in the photopeak projections, the number of scatter counts is determined using the ollowing equation
Cscat ~ (Clower/Ws + Cupper/Ws)*Wm/2
where
Clower = counts in left window
Cupper = counts in right window
Ws = width of left and right scatter windows (keV)
Wm = width of photopeak window (keV)
Cscat = number of scatter counts
Phantom studies and computer simulations performed by Ogawa et al and Ichihara et al2 showed the method could estimate the number of scattered photons fairly accurately. However, neither paper addresses the potential for increased noise when the scatter counts are subtracted.
A Monte Carlo investigation of the method by Ljungberg et al3 questioned the use of the upper (right) scatter window noting that using the right scatter window might make the TEW method more susceptible to noise. When only the lower energy window is used, Clower = 0 and the estimated number of scatter counts becomes
Cmax = (Clower*Wm)/(2*Ws)
Monte Carlo simulations performed by Buvat et al4 demonstrated an 18% overestimate of the scatter counts when both scatter windows were used, and a 14% underestimate of the scatter counts when only the lower scatter window was used. Good relative activity quantification was also demonstrated by the simulations when only the lower window was used.
Equipment
- Jaszczak phantom with cold rods and spheres
- ACNP kidney phantom
- Picker PRISM 3000 triple head SPECT camera
Method
A standard Jaszczak phantom containing the solid rod and spheres inserts was filled with water and 1110 MBq (30 mCi) of Tc-99m. A SPECT acquisition was acquired using three energy windows
- Photopeak – 15% window centered at 140 keV.
- Scatter1 – 3 keV window centered at 126 keV.
- Scatter2 – 3 keV window centered at 153 keV.
with these acquisition parameters: 120 projections, 30 s/projections, 3 degrees/projections and a 128×128 projection matrix. The images from each energy window was stored in a separate file.
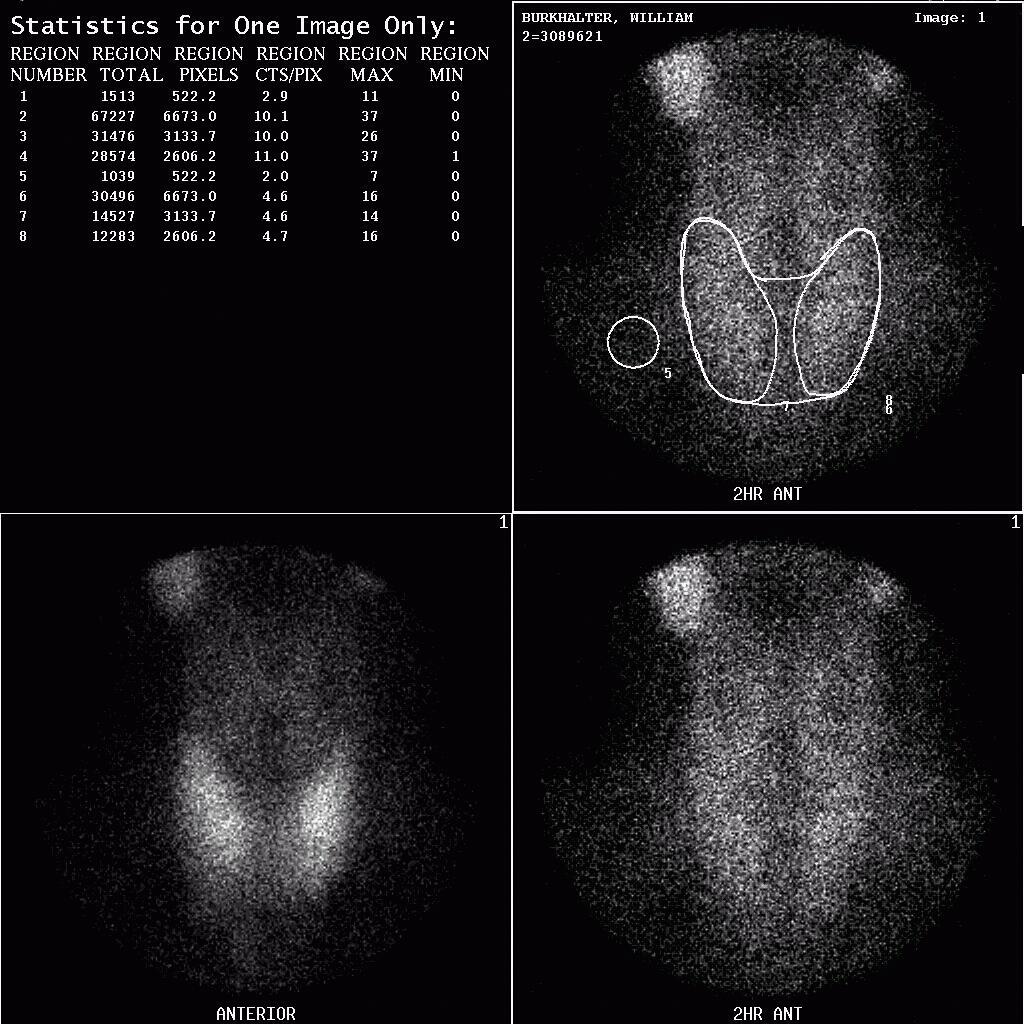
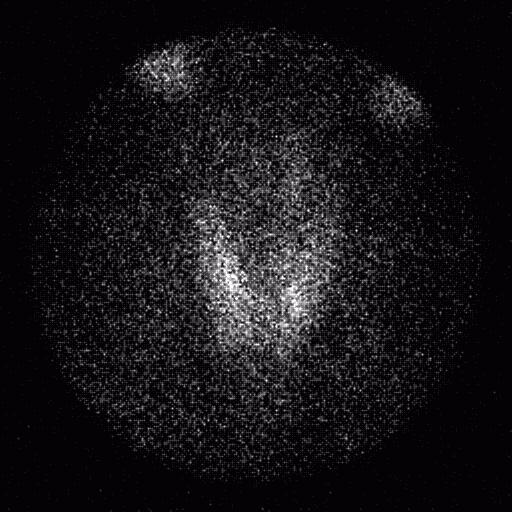
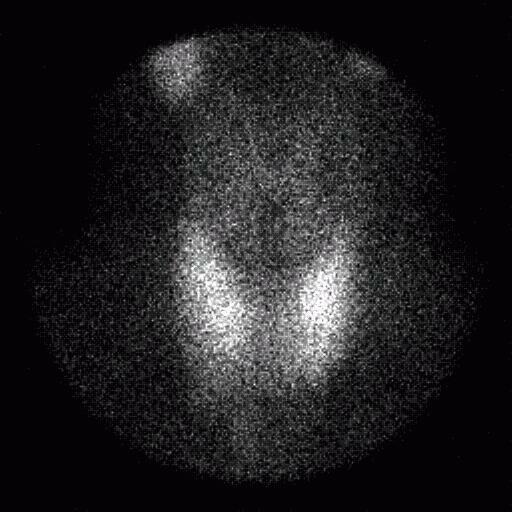
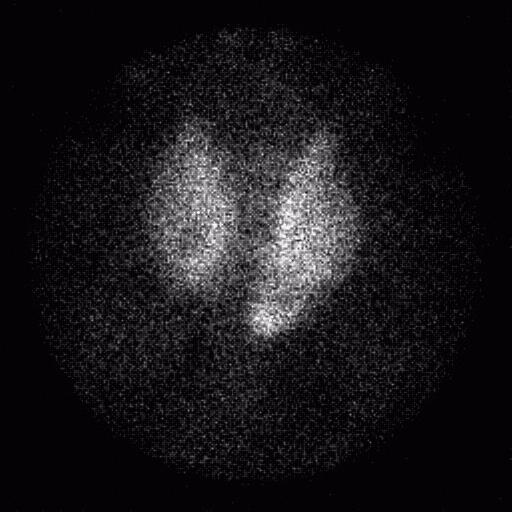
The ACNP (American College of Nuclear Physicians) kidney phantom consists of two fillable kidney objects with a cold spot defect located in the middle of the right kidney and superior portion of the left kidney. The objects were filled with approximately 370 MBq (10 mCi) of Tc-99m and placed within a water bath. A SPECT acquisition was acquired using the same energy windows used for the Jaszczak phantom, 120 projections, 30 s/projection, 3 degrees/projection and a 128×128 projection matrix.
Processing
Once the acquisition is complete, there are three files, the photopeak projections, and two scatter projections. The TEW method was performed two different ways; with both scatter windows (TEW2) and with just the lower scatter window (TEW1) as suggested by Ljungberg et al. All image processing was done through the Odyssey software.
TEW2
- Divide the counts in each pixel of the scatter projections by the width in keV of the scatter window.
- Add the two scatter window projections together.
- Multiply the resultant projections by half of the photopeak window width in keV.
- Subtract the result from the original projections.
TEW1
- Divide the counts in each pixel of the scatter projections from the lower window by the width in keV of the scatter window.
- Multiply the resultant projections by half the photopeak window width in keV.
- Subtract the result from the original projections.
With the window settings used in this experiment (3 keV scatter windows, 15% (21 keV) photopeak window), the scatter correction using both lower and upper scatter windows was performed by adding the scatter projections together and multiplying the result by a factor of 3.5. The scatter projections were then subtracted from the main photopeak projections. For the TEW1 method, the pixels of the lower scatter window projections were scaled by a factor of 3.5 and subtracted from the photopeak window projections
Reconstruction
A 3 pixel thick slice was reconstructed with a ramp filter through the center of the spheres from each of the corrected and uncorrected projections. To investigate uniformity and noise, a 9 pixel thick slice was reconstructed with a ramp filter through the uniform water section of the phantom. Attenuation correction was applied using the system attenuation correction software and an attenuation coefficient of 0.11 cm-1. No additional postfilters were applied to the reconstructed images.
The ACNP kidney phantom was reconstructed using a ramp filter and single pixel thick slices, and then filtered using a Wiener 3-D postfilter. The filtered images were reformatted to 3 pixel thick coronal and transverse slices. No attenuation correction was applied to the phantom images.
Results and Discussion
The total counts per projection in the ACNP kidney phantom images ranged from 15-20 kcounts in the photopeak window, 1.5-2.6 kcounts in the lower scatter window and 300-600 counts in the upper scatter window. The count loss when both scatter windows were used to estimate the scatter counts in the photopeak window was almost 50% (6-10 kcounts/projection subtracted from the photopeak projections) and around 35% (5-9 kcounts/projection subtracted) when only the lower scatter window was used.
Circular ROIs was drawn through the each sphere of the Jaszczak phantom to obtain the mean counts/pixel within the sphere for the corrected and uncorrected images. The same ROIs were used to obtain the mean counts/pixel from the center of the phantom. The percent contrast for each sphere was calculated using the equation
% Contrast = (bkg counts – ROI counts)/bkg counts
| % Contrast | |||
|---|---|---|---|
| Uncorrected | 2 Window | 1 Window | |
| 36 mm | 80 | 90 | 92 |
| 31 mm | 66 | 75 | 83 |
| 25 mm | 52 | 68 | 69 |
| 19 mm | 49 | 60 | 65 |
| 15 mm | 23 | 41 | 34 |
The most noticeable problem with the scatter corrected images is a significant decrease in counts and increase in noise. The inherent noisiness of the ramp filter also contributes to the noise in the reconstructed images. An interesting item to note is the improved contrast when only the single window is used compared to when both windows are used. This suggests that using only the lower scatter window may produce better results as suggested by Ljungberg et al3. Neglecting the higher scatter window for the higher energy photopeak is also recommended by Ogawa to avoid increasing statistical noise.
The additional noise introduced by the TEW scatter correction may be compensated for somewhat by applying a different filter to the projections or postfiltering the reconstructed images.
The slices from the uniform section of the phantom was used to determine the uniformity and noise of the corrected and uncorrected slices. The mean, standard deviation, maximum and minimum counts per pixel from a 15×15 pixel ROI were used to calculate the integral uniformity and the RMS noise level for the uniform section. The relatively high uniformity values are a result of the ramp filter which is inherently noisy.
| Mean cts/pix | Max cts/pix | Min cts/pix | Int Unif |
|---|---|---|---|
| 19619 | 24975 | 14598 | 26.2% |
| 19750 | 23922 | 16182 | 19.3% |
| 19134 | 24246 | 14715 | 24.5% |
| 19371 | 24336 | 14940 | 23.9% |
| 18785 | 23391 | 14598 | 23.1% |
| 19331.8 | 24174 | 15006.6 | 23.4% |
When both the upper and lower energy windows are used the integral uniformity increases significantly. This a result of the subtraction of the scatter counts from the projections.
| Mean cts/pix | Max cts/pix | Min cts/pix | Int Unif |
|---|---|---|---|
| 11789 | 20709 | 5661 | 57.1% |
| 12451 | 18261 | 7326 | 42.7% |
| 12139 | 17955 | 5661 | 52.1% |
| 12391 | 18513 | 6084 | 50.5% |
| 11921 | 20781 | 6039 | 55.0% |
| 12138.2 | 19243.8 | 6154.2 | 51.5% |
Using a just the lower energy window to estimate the scatter improves the integral uniformity slightly, although the values remain relatively high compared to the uncorrected images.
| Mean cts/pix | Max cts/pix | Min cts/pix | Int Unif |
|---|---|---|---|
| 13551 | 21951 | 67.32 | 53.1% |
| 13896 | 19827 | 8163 | 41.7% |
| 13227 | 21870 | 6732 | 52.9% |
| 13999 | 19611 | 7785 | 43.2% |
| 13512 | 20601 | 7614 | 46.0% |
| 13637 | 20772 | 7405.2 | 47.4% |
The noise level in the reconstructed images can be largely alleviated by filtering the images as is commonly done with clinical studies. However, the uniformity of the scatter corrected filtered images will still be greater than the uncorrected images simply because of the loss of counts incurred when the estimated scatter counts are subtracted.
A Wiener 3-D postfilter was applied to the same images resulting in a significant improvement in image noise. However, the scatter corrected filtered images still demonstrated greater non-uniformity and appeared noisier than the uncorrected filtered images.
Evaluation of the ACNP kidney phantom was performed qualitatively on both the filtered and unfiltered images. In all images, both defects were clearly visualized, although the scatter corrected images showed a lower count density and noise was increased significantly. Applying a Wiener 3-D postfilter improved the appearance of the images considerably.
Conclusion
The triple energy window scatter correction method evaluated using the Jaszczak and ACNP kidney phantoms showed decreased count images and increased noise, although contrast was improved. The phantom studies suggest that the triple energy window method may not be well suited for studies involving large distributions of radioactivity such as brain or liver studies because of the poor noise and uniformity properties. This scatter correction method may prove to be more applicable to studies involving smaller discrete radioactivity distributions such as cardiac or renal studies. Planar studies may also benefit from this correction method, although it was not investigated here. The ramp filter used to reconstruct the corrected and uncorrected images is inherently noisy, so the use of more optimal filters in addition to or instead of the ramp filter when reconstructing images should be considered. Further research using more clinically relevant phantoms is needed to further evaluate the noise and uniformity properties of the TEW method.
Bibliography
- Ogawa K, Harata Y, Ichihara T, Kubo A, Hashimoto S, A practical method for position dependent Compton scatter correction in single photon emission CT, IEEE Trans Nucl Med, 10:408-412 1991
- Ichihara T, Ogawa K, Motomura N, Kubo A, Hashimoto S, Compton scatter compensation using the triple-energy window method for single and dual isotope SPECT, J Nucl Med 34:2216-2221 1993
- Ljungberg M, King MA, Hademenos GJ, Strand SE, Comparison of four scatter correction methods using Monte Carlo simulated source distributions, J Nucl Med 35:143-151 1994
- Buvat I, Rodrigues-Villafuerte M, Todd-Pokropek A, Benali H, Di Paola R, Comparative assessment of nine scatter correction methods based on spectra analysis using Monte Carlo simulations, J Nucl Med 36:1476-1488 1995